Reliance / Single IRB of Record
Reliance Overview
Reliance agreements allow for a Federalwide Assurance (FWA) covered institution to provide IRB oversight on behalf of another institution or individual. This process can be a helpful tool for investigators to streamline collaborative research, as their protocol will only have to be reviewed in its entirety by one institution’s IRB. Reliance agreements are created on a case-by-case basis and are applicable only to a single study per agreement.
GU as Relying IRB
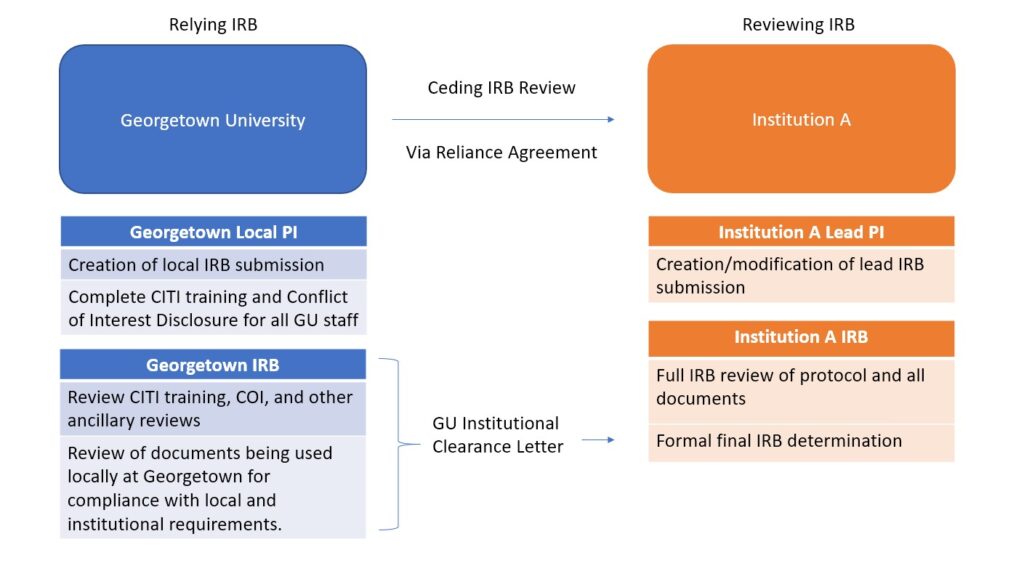
By ceding IRB review, Georgetown IRB does not complete a full IRB review and instead relies on the determination made by the reviewing IRB. However, Georgetown IRB maintains several responsibilities of local consideration review. Upon completion of all local consideration reviews, Georgetown IRB will provide a Georgetown Institutional Clearance Letter to demonstrate to the reviewing IRB that all local review has been completed. Upon approval of the study by the reviewing IRB, the Georgetown Local PI will be able to begin to conduct the study.
Examples of instances where it may be appropriate to rely on another institution:
- Georgetown PI is a collaborator on study primarily being executed at a different institution
- Georgetown is a participating site of a multi-site clinical trial
- Georgetown PI wants to still be engaged in a project from a previous affiliation
Georgetown University IRB is willing to rely on another FWA-covered institution for appropriate collaborative or multi-site research.
GU as Reviewing IRB
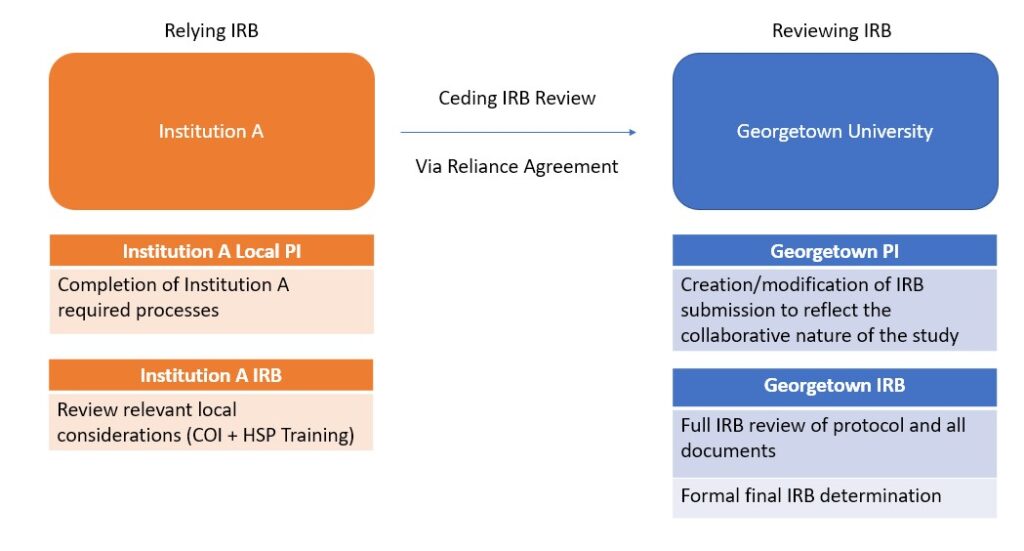
Georgetown University IRB can act as a reviewing IRB for FWA-covered institutions for collaborative research only. As the reviewing IRB, Georgetown will provide IRB review and regulatory oversight on behalf of a collaborating institution. Please note that the collaborating institute may have local requirements required by their IRB.
Example of instances where it may be appropriate to have Georgetown IRB review on behalf of another institution:
- Collaborating institution is only executing a portion of the entire protocol
- Subjects are only being recruited and consented at Georgetown*
- Previous study team member is no longer with Georgetown and is now affiliated with another institution.
To request Georgetown to be a reviewing IRB for collaborative research please complete: Reliance Request Form.
In certain circumstances, Georgetown IRB can enter a reliance agreement with an individual investigator. This may be appropriate when an individual is not affiliated with an FWA-covered institution. Under this Individual Investigator Agreement (IIA), Georgetown IRB would provide IRB review and regulatory oversight over this individual. This is reviewed on a case-by-case basis. Please see: Adding an External Member to an IRB Submission
How to Establish a Reliance Agreement
If a reliance agreement appears appropriate for your study, please first complete the following Reliance Request Form. This will allow Georgetown IRB to conduct a preliminary review to determine if a reliance agreement is appropriate for your study. If deemed appropriate, you will receive a “Reliance Confirmation Letter” to be attached to your IRB submission.
For more detailed information surrounding the use of an External IRB
Types of Reliance Agreements
Once Georgetown IRB has determined reliance appropriate, a reliance agreement must be fully executed by an institutional official from each institution. As part of the Reliance Request Form, you will be asked which reliance agreement you would like to use.
The IRB Authorization Agreement (IAA) is the standard paper form agreement used at Georgetown. This document should be filled out by the Georgetown PI. This IAA can then be provided to the external institution for partial execution by the institution’s Signatory Official. Once partially executed, this document can be returned to the Georgetown IRB for full execution.
Alternatively, Georgetown University is also a participant in the SMART IRB platform, which can be used to execute a reliance agreement.
Additionally, Georgetown University has executed Master Reliance Agreements with the following institutions. A reliance agreement is not required for these institutions.
Please note regardless of which reliance agreement is being used, the reliance request form should still be filled out and “Reliance Confirmation Letter” obtained.
Single IRB (sIRB) Requirements
Single IRB (sIRB) refers to the policy of a single IRB conducting ethical review on behalf of all participating sites for multi-site studies.
As of January 25, 2018, the National Institutes of Health (NIH) requires all wholly or partially funded multi-site studies where each site will conduct the same protocol to use an sIRB.
While Georgetown University can cede review to an sIRB, Georgetown cannot act as the sIRB for a multi-site study.
If you have a NIH-funded multi-site study, consider the following options:
- Request for one of the participating site’s IRB to act as the sIRB for the study
- Contract a commercial IRB to act as the sIRB for the study